Research Article
Intravitreal ranibizumab in the management of acute central serous Chorioretinopathy

Ibrahim Nawaiseh1, Ahmad Halawa2* and Dina Alardah2
1MD, Department of Ophthalmology, Ibn Al-Haytham Hospital and Associate Professor at King Hussein Cancer Center, Amman, Jordan
2MD, Department of Ophthalmology, Ibn Al-Haytham Hospital, Amman, Jordan
*Address for Correspondence: Ahmad Halawa, MD, Department of Ophthalmology, Ibn Al-Haytham Hospital, Amman, Jordan, Email: [email protected]
Dates: Submitted: 13 November 2017; Approved: 22 November 2017; Published: 24 November 2017
How to cite this article: Nawaiseh I, Halawa A, Alardah D. Intravitreal ranibizumab in the management of acute central serous Chorioretinopathy. Int J Clin Exp Ophthalmol. 2017; 1: 049-054. DOI: 10.29328/journal.ijceo.1001007
Copyright Licence: © 2017 Nawaiseh I, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Acute central serous chorioretinopathy; Ranibizumab
Abstract
Purpose: To evaluate the efficacy of ranibizumab in hastening the recovery of acute CSCR when given immediately at time of diagnosis.
Methods: In This retrospective case series, a total of 72 patients diagnosed with acute CSCR where reviewed, of which 63 received Ranibizumab at presentation. The patients were evaluated using Best corrected visual acuity, Ophthalmic examination, Optical coherence tomography (OCT) and fluorescein angiography, in addition to indocyanine green angiography and OCT angiography in some cases, at presentation, one week, one month and two months’ post injection.
Results: From the total 72 patients diagnosed with acute CSCR, 63 of them received intravitreal ranibizumab and the remaining 9 patients preferred to go for observation. The mean age of patients was 41.2 year old. The ratio of male to female was 8:1. The mean BCVA at presentation was 6/15 on Snellen chart. All patients who received ranibizumab injection showed an improvement after 1 week, with a mean improvement in BCVA of two lines. Of them, 43 patients were back to BCVA of 6/6 after 2 months and showed complete resolution of sub retinal fluid. The remaining 20 patients showed an additional mean of improvement of one line (over the previous two lines) after the 2 months.
Conclusion: Intravitreal ranibizumab hasten the recovery of both the BCVA and central macular thickness on OCT in acute CSCR when given immediately at presentation.
Introduction
CSCR is a relatively rare condition typically occurring in young adults, especially males in their twenties to fifties, who exhibit acute or subacute central visual loss or distortion. It is an idiopathic disorder characterized by a localized serous detachment of the sensory retina at the macula secondary to leakage from the choriocapillaris in association with retinal pigment dysfunction, yet the exact pathophysiology is not well understood [1,2]. Different treatment options are available including Observation, photodynamic therapy with vertaporfin, laser photocoagulation, pharmacotherapy with adrenergic blockers and anti-corticosteroids like Ketoconazole, micropulse diode laser and intravitreal anti VEGF agents have been used. None of them is considered the mainstay treatment of CSCR, which reflects the controversy of this disease [3-5]. Ranibizumab (Lucentis) is a recombinant humanized IgG1 monoclonal antibody fragment that binds to and inhibits vascular endothelial growth factor A, thus interrupts the interaction of vascular endothelial factor with its receptors which prevents the subsequent growth of new blood vessels and stabilizes the blood retinal barrier. It is used in the treatment of many retinal pathologies. In this study, we evaluated the efficacy of ranibizumab in early management of acute CSCR.
Methods
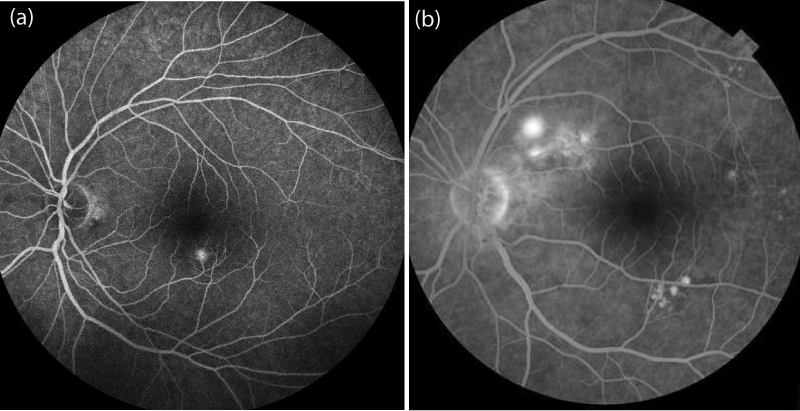
In this retrospective study, 72 patients who were diagnosed with acute CSCR between January 2014 and May 2017 were reviewed. The diagnosis of CSCR was established by dilated fundus examination, optical coherence tomography (OCT) and fluorescein angiography (FA), in addition to indocyanine green angiography and OCT angiography in some cases (to rule out choroidal neovascularisation and polypoidal choroidal vasculopathy). Inclusion criteria of this study were: sub retinal foveal fluid evident on OCT (Figures 1,2) and typical leakage pattern on FA (Figure 3). Patients who had a history of previous treatment for CSCR, such as laser photocoagulation or photodynamic therapy (PDT), and a history of intraocular surgery or vitreoretinal intervention, including retinal laser therapy, were excluded.
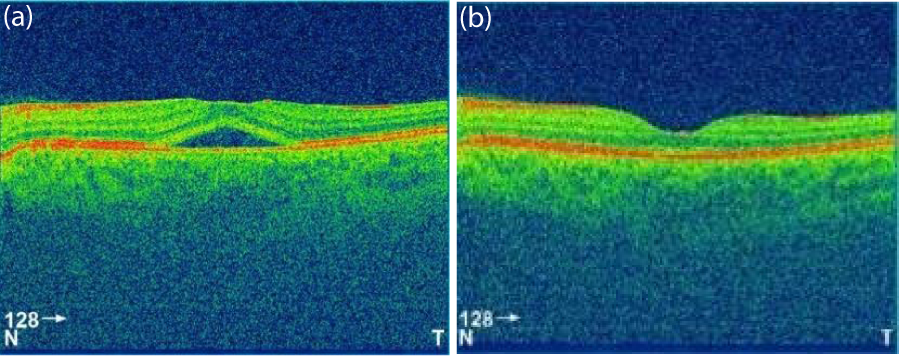
Figure 1: OCT of one patient at presentation (a) and at 1 month (b). The figure shows the resolution of retinal detachment.
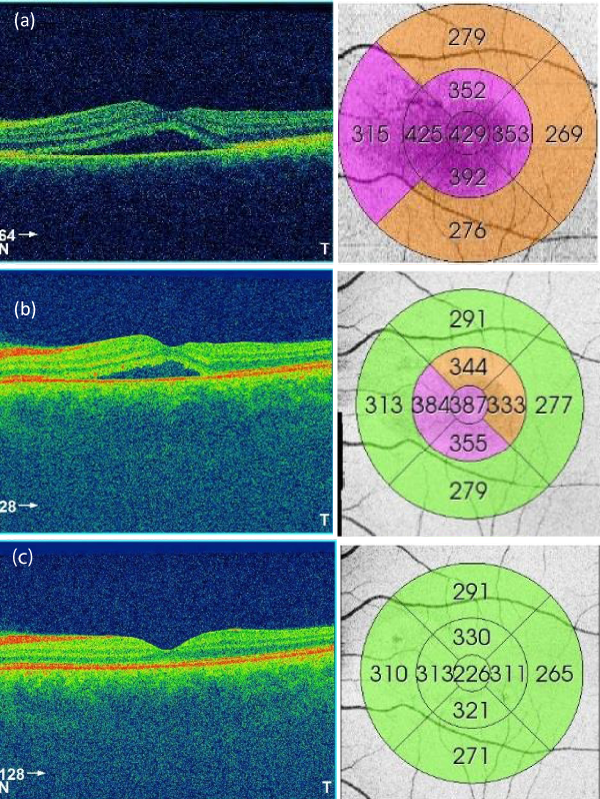
Figure 2: OCT and thickness map of a patient at presentation (a), at 1 week (b), and at 2 months (c). The figure shows the improvement of central foveal thickness and eventual resolution of sub retinal fluid.
All patients were informed about the different treatment possibilities including observation. They were also informed about the possible advantages and complications of the use of intravitreal injections, and were informed about the off-label nature of this treatment. All patients received an intravitreal injection of Ranibizumab in the operation room under complete aseptic conditions. Topical anesthetic drops were given first, and then a lid speculum was inserted. After cleaning the injection site with 5% povidone-iodine, the needle was inserted through the pars plana, and 0.05 ml (0.5 mg) of ranibizumab was injected.
All patients were followed up at one week, one month and 2 months’ post injection. At follow up visits, BCVA, slit lamp examination, dilated fundus examination and OCT were performed to all patients. All finding at baseline and each follow up visit were reported and compared.
Results
From the total 72 patients diagnosed with acute CSCR, 63 of them received intravitreal ranibizumab and the remaining 9 patients preferred to go for observation. The mean age of patients was 41.2 year old. The ratio of male to female was 8:1. The mean BCVA at presentation was 6/15 on Snellen chart. All patients who received ranibizumab injection showed visual improvement after 1 week, with a mean improvement in BCVA of two lines. 68% of patients were back to BCVA of 6/6 after 2 months and showed complete resolution of sub retinal fluid (Figure 2). The remaining patients showed an additional mean of improvement of one line (over the previous two lines) after the 2 months.
None of the eyes had decreased vision compared with the baseline BCVA. There was a significant decrease in the mean of central corneal thickness throughout the study, which was correlated with the improvement in both of BCVA and FA leakage.
Discussion
CSCR is a benign self-limited condition in which idiopathic detachment of the neurosensory retina occurs over an area of leakage from the choriocapillaris through the RPE [3-5]. Studies suggest an annual incidence rate of 10 per 100,000 in men, with CSCR occurring 6 times more commonly in men compared with women. A number of hypotheses for the pathophysiology have incorporated abnormal ion transport across the RPE and focal choroidal vasculopathy [6]. Indocyanine green angiography has demonstrated evidence of choroidal lobular ischemia, choroidal venous congestion and multiple areas of vascular hyperpermeability. RPE damage may also occur by shedding of outer photoreceptor segments with a primarily intact blood-retinal barrier and result in accumulation of fluid in sub retinal space [7].
There is no established treatment for CSCR. Many treatment modalities have been described to manage CSCR, such as observation, PDT [9,10], focal laser photocoagulation [11], adrenergic blockers, anti-corticosteroids like Ketoconazole, micropulse diode laser and anti-VEGF injections. The high spontaneous remission rate (approximately 90%) favors conservative management and lifestyle counseling as first-line of therapy. However, observation alone is usually associated with longer recovery time (up to 80 days) and higher chance of recurrence [12,13]. There are some studies supporting the benefit of early treatment of CSCR in which they proposed that the potential advantage is hastening the recovery which is mediated by a lower rate of RPE degeneration in treated eyes. Many studies have demonstrated faster resolution of sub retinal fluid in patients who underwent laser photocoagulation compared to control eyes [14-16].
However, there are complications associated with laser photocoagulation in which it may induce scotoma formation in the central visual field through RPE damage at the macula. Also, it is thought that laser photocoagulation does not influence the final visual outcome or rate of recurrence. Photodynamic therapy (PDT) with verteporfin has been also used to treat CSCR. It is believed that PDT works in CSCR by inducing choroidal hypoperfusion, and vascular narrowing and remodeling to negate choroidal hyperpermeability which is consistently found in CSCR cases [17,18]. Maruko et al. found that subfoveal choroidal thickness and ICG hyperpermeability decreased after PDT compared to laser photocoagulation [19]. PDT necessitates the avoidance of physical activity after treatment and is associated with high cost and there are still concerns about possible late-onset macular damage, such as RPE changes, excessive choriocapillaris hypoperfusion, and secondary choroidal neovascularization. Therefore, this modality should be used with caution since eyes with CSCR have a high potential for spontaneous recovery [20,21].
Another reported treatment modality for CSCR is anti-corticosteroids. The suggestion of using anti-corticosteroids, like ketoconazole, as a treatment is due to the proposed relation of corticosteroid use and the development of CSCR [22]. This has led to several clinical trials to assess the effect of such treatment.
Golshahi, in a comparative non-randomized study of 15 patients with acute CSCR treated with ketoconazole for 4 weeks versus 15 patients in the control group, concluded that systemic ketoconazole was not associated with a significantly better outcome [23]. VEGF is a regulator of ocular angiogenesis and vascular permeability. It is produced by retinal and choroidal cells in response to ischemia and has been implicated as the major mediator of vascular hyperpermeability. VEGF results in increased vascular permeability and edema by uncoupling endothelial cell-to-cell junctions.
In CSCR there is increased choroidal hyperpermeability claimed to be due to increased VEGF. Thus, it seems logic to treat such cases with anti VEGF [24,25]. Intravitreal injection of bevacizumab, a VEGF antibody with antipermeability properties, was reported to be associated with visual improvement and reduced neurosensory detachment without adverse events in patients with CSCR [26]. A newer anti VEGF agent is ranibizumab, which has several claimed advantages over bevacizumab. Considering the molecular weight of each medication (ranibizumab is a 48-kDa Fab fragment, whereas bevacizumab is a complete 149-kDa antibody), ranibizumab may be more effective for treating CSCR because of its smaller molecular weight and possible deeper penetration to choroidal vascular hyperpermeability lesions in patients with CSCR [27]. Ranibizumab is a recombinant humanized IgG1 monoclonal antibody fragment that binds to and inhibits vascular endothelial growth factor A, thus interrupts the interaction of vascular endothelial factor with its receptors [28]. Ranibizumab is injected intravitreally as treatment in many retinal pathologies like exudative age-related macular degeneration, diabetic macular edema, and macular edema secondary to central and branch retinal vein occlusion [29]. In our study, we would like to show that Intravitreal injection of ranibizumab may be an effective and safe treatment option for acute CSCR.
Our results demonstrated that, Intravitreal injection of ranibizumab in acute CSCR is associated with rapid resolution of neurosensory retinal detachment with a reduction of central foveal thickness and an increase in visual acuity. Intravitreal injection of ranibizumab appeared to be superior in hastening the recovery compared to observation alone. It is also, associated with a less side effects and complications compared to the other treatment modalities for CSCR. In a randomized, controlled, and prospective study, Kim et al. reported effectiveness of Intravitreal injection of ranibizumab for acute CSCR (symptomatic for less than 3 months) on 20 patients (0.5 mg/0.05 ml) who were followed up for 6 months [30]. They reported longer time required for complete resolution of neurosensory retinal detachment in the observation group when compared with the Intravitreal injection of ranibizumab group, but they also found no statistically significant difference between the groups at the sixth month. Schaal et al. [31], treated 12 eyes with CSCR in which patients received 2±1 intravitreal injections of bevacizumab (2.5 mg) on average during a followup of 24±14 weeks. Mean BCVA increased by 2±2 lines; The change in BCVA was significant (P<0.02). Mean central corneal thickness improved significantly over followup (P<0.05), with six patients (50%) showing complete resolution of subretinal fluid. In summary, we found that there is significant improvement in the speed of recovery regarding BCVA and central macular thickness in patients with acute CSCR when given Intravitreal ranibizumab upon diagnosis.
References
- Gass JD. Pathogenesis of disciform detachment of neuroepithelium. II. Idiopathic central serous choroidopathy. Am J Ophthalmol. 1967; 63: 587-615. Ref.: https://goo.gl/XRKjVm
- Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin. Exp. Ophthalmol. 2013; 41: 201-214. Ref.: https://goo.gl/hpLwqg
- Brancato R, Scialdone A, Pece A, Coscas G, Binaghi M. Eight-year follow-up of central serous chorioretinopathy with and without laser treatment. Graefes Arch Clin Exp Ophthalmol. 1987; 225: 166-168. Ref.: https://goo.gl/UVqrUz
- De Laey JJ. Central serous chorioretinopathy: to treat or not to treat?. Doc Ophthalmol. 1986; 61: 367-372. Ref.: https://goo.gl/SpfSyy
- Ficker L, Vafidis G, While A, Leaver P. Longterm followup of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1988; 72: 829-834. Ref.: https://goo.gl/kXUGNL
- Spitznas M. Pathogenesis of central serous retinopathy: a new working hypothesis. Graefes Arch Clin Exp Ophthalmol. 1986; 224: 321-324. Ref.: https://goo.gl/qcjFhL
- Spaide RF, Hall L, Haas A, lisa MD, laura BS, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996; 16: 203-213. Ref.: https://goo.gl/RxQU5w
- Chan WM, Lai TY, Lai RY, Liu DT, Lam DS. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology. 2008; 115: 1756-1765. Ref.: https://goo.gl/jj4eZC
- Yannuzzi LA, Slakter JS, Gross NE, Huang, Klancnik, et al. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2003; 23: 288-298. Ref.: https://goo.gl/jxcbx2
- Burumcek E, Mudun A, Karacorlu S, Arslan MO. Laser photocoagulation for persistent central serous retinopathy: results of longterm follow-up. Ophthalmology. 1997; 104: 616-622. Ref.: https://goo.gl/EDShsv
- Loo RH, Scott IU, Flynn HW Jr, Gass JD, Murray TG, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002; 22: 19-24. Ref.: https://goo.gl/4oNHPd
- Wang M, Munch IC, Hasler PW, Pru¨nte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol. 2008; 86: 126-145. Ref.: https://goo.gl/u4GGU8
- Robertson DM, Ilstrup D. Direct, indirect, and sham laser photocoagulation in the management of central serous chorioretinopathy. Am J Ophthalmol. 1983; 95: 457-466. Ref.: https://goo.gl/2A8trZ
- Leaver P, Williams C. Argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1979; 63: 674-677. Ref.: https://goo.gl/ojA3fi
- Ficker L, Vafidis G, While A, Leaver P. Long-term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1988; 72: 829-834. Ref.: https://goo.gl/4gJ8H9
- Chan WM, Lai TY, Lai RY, Liu DT, Lam DS. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology. 2008; 115: 1756-1765. Ref.: https://goo.gl/idr9RM
- Schmidt-Erfurth U, Laqua H, Schlötzer-Schrehard U, Viestenz A, Naumann GO. Histopathological changes following photodynamic therapy in human eyes. Arch Ophthalmol. 2002; 120: 835-844. Ref.: https://goo.gl/pEUA14
- Maruko I, Iida T, Sugano Y, Ojima A, Ogasawara M, et al. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010; 117: 1792-1799. Ref.: https://goo.gl/od6wv5
- Cardillo Piccolino F, Eandi CM, Ventre L, Rigault de la Longrais RC, Grignolo FM. Photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2003; 23: 752-763. Ref.: https://goo.gl/A9TAs4
- Chan WM, Lam DS, Lai TY, Tam BS, Liu DT, et al. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003; 87: 1453-1458. Ref.: https://goo.gl/vSk3DX
- Jampol LM, Weinreb R, Yannuzzi L. Involvement of corticosteroids and catecholamines in the pathogenesis of central serous chorioretinopathy: a rationale for new treatment strategies. Ophthalmology. 2002; 109: 1765-1766. Ref.: https://goo.gl/s6HKSQ
- Golshahi A, Klingmüller D, Holz FG, Eter N. Ketoconazole in the treatment of central serous chorioretinopathy: a pilot study. Acta Ophthalmol. 2010; 88: 576-581. Ref.: https://goo.gl/yyTaZF
- Torres-Soriano ME, Garcia-Aguirre G, Kon-Jara V, Ustariz-Gonzáles O, Abraham-Marín M, et al. A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy (case reports). Graefes Arch Clin Exp Ophthalmol. 2008; 246: 1235-1239. Ref.: https://goo.gl/CdKEPD
- Wu L, Martinez-Castellanos MA, Quiroz-Mercado H, Arevalo JF, Berrocal MH, et al. Twelve-month safety of intravitreal injections of bevacizumab (Avastin): results of the Pan-American Collaborative Retina Study Group (PACORES). Graefes Arch Clin Exp Ophthalmol. 2008; 246: 81-87. Ref.: https://goo.gl/k8uh4o
- Torres-Soriano ME, Garcia-Aguirre G, Kon-Jara V, Ustariz-Gonzáles O, Abraham-Marín M, et al. A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy (case reports). Graefes Arch Clin Exp Ophthalmol. 2008; 246: 1235-1239. Ref.: https://goo.gl/YyULAK
- Gomi F, Tano Y. Polypoidal choroidal vasculopathy and treatments. Curr Opin Ophthalmol. 2008; 19: 208-212. Ref.: https://goo.gl/Kmx17h
- Lantry LE: Ranibizumab, a mAb against VEGF-A for the potential treatment of agerelated macular degeneration and other ocular complications. Curr Opin Mol Ther. 2007; 9: 592-602. Ref.: https://goo.gl/t4EfvY
- Brown DM, Campochiaro PA, Singh RP, Li Z, Grav S, et al.Ranibizumab for macular edema following central retinal vein occlusion: sixmonth primary end point results of a phase III study. Ophthalmology. 2010; 117:1124-1133. Ref.: https://goo.gl/sJhHVG
- Kim M, Lee SC, Lee SJ. Intravitreal ranibizumab for acute central serous chorioretinopathy. Ophthalmologica. 2013; 229: 152-157. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/23257663
- Schaal KB, Hoeh AE, Scheuerle A, Schuett F, Dithmar S, et al. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol. 2009; 19: 613-617. Ref.: https://goo.gl/g832H7