More Information
Submitted: 27 April 2020 | Approved: 21 May 2020 | Published: 22 May 2020
How to cite this article: Sandeep CS, Vijayakumar N, Sukesh Kumar A. Can we predict Alzheimer’s Disease through the eye lens? Int J Clin Exp Ophthalmol. 2020; 4: 038-040.
DOI: 10.29328/journal.ijceo.1001031
Copyright Licence: © 2020 Del Sandeep CS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Alzheimer’s Disease; Early diagnosis; Retina; OCT
Can we predict Alzheimer’s Disease through the eye lens?
Sandeep CS1*, Vijayakumar N2 and Sukesh Kumar A2
1Research Scholar, College of Engineering, University of Kerala, Trivandrum, India
2Research Guide, College of Engineering, University of Kerala, Trivandrum, India
*Address for Correspondence: Sandeep CS, Research Scholar, College of Engineering, Trivandrum, University of Kerala, India, Email: [email protected]
Alzheimer’s Disease (AD) is a common dementia problem of the old population. The two main hallmarks of AD are tau protein and amyloid-beta protein. The relevant investigations on AD suggest that these proteins are also seen in the eye. There are many tests and imaging modalities are used for AD diagnosis. But these techniques are still unable to predict the disease effectively. In this regard, the lens of the eye may help in diagnosing AD. Therefore, a reliable technique for measuring the lens or retina must be selected. In this paper, we focus on the different types of retinal diseases occur in AD patients and the use of the Optical Coherence Tomography (OCT) technique is used for diagnosing AD.
Alzheimer’s disease (AD) is a type of dementia destroying the old age population around the globe. If the progression of AD continuous like this, millions of people will be in big trouble [1]. The early diagnosis of the disease can control disease progression. Therefore, a new technique should be developed more economically to meet the expense of AD subjects and their caretakers. As AD is a brain-related disease, visual problems have also been identified in the early stages of AD. Visual function loss other than perception may be the first sign of AD [2]. The researchers are starting that before going to brain analysis, eye analysis can be used because AD may start in the portion of the brain that integrates visual function with other areas of the brain, the visual association area. Hence, it is easy to find treatment plans in detecting early AD. For an effective and early diagnosis of AD, a population-based study is necessary and required, which gives an idea about the various tests involved in determining AD. In this paper, we focus on a different eye-related disease that occurs in AD patients.
AD and eye related disorders
Open-Angle Glaucoma (OAG) is a type of eye disease in AD subjects. In OAG, the angle of the eye in which the iris connects the cornea is wide as well as open, but the canals of the eye will be congested leading to rise in internal eye pressure and consequent injury to the optic nerve. It is the most common type of glaucoma, affecting about four million Americans, from different experiments, it is clear that the prevalence of probable glaucoma in AD patients is significantly higher than that of normal. It is then leading to visual impairment and is the second leading cause of blindness in the United States. Glaucoma is a prolonged and deteriorating eye disease in AD by the loss of retinal cells [3]. Amyloidopathy is another eye disease that affects the AD subjects due to presence of amyloid beta protein in the eye. Emerging studies show the connection of amyloidopathy in several eye disorders related to AD [4]. Age-related macular degeneration (AMD) is a prominent eye disease affecting the old age and cause blindness to AD patients [5]. AMD is pathologically categorized by the existence of drusen, which are deposits that occur outside the retinal cells and damages the macula. Cataract is another common eye disorder with aging that may affect the AD population. It forms a cloudy appearance in the lens that avoids the transfer of images to the retina. Thus the retina cannot send the signals to the brain. Pupillary function and AD suggests that there are defects in the pupil of AD patients [6]. Ocular AD-related histopathology proposes that there is variation in the eye lens according to the AD pathology. This leads to retinal cell degeneration of AD patients that has been recognized by several histopathological studies [7,8].
AD and OCT
From the above section, it is clear that there are different eye diseases related to AD. Therefore, different differentiating AD disease from disease progression is a complex task. In this scenario, retina images are carefully taken from a reliable device for diagnosing AD [9]. In AD patients, retinal nerve fiber loss (RNFL) occurs in the early stage of AD. Accordingly, a retinal imaging technique is to wisely chosen for the task. For this purpose, Optical Coherence Tomography (OCT) imaging technique that can be used for examining the RNFL region of the retina [10]. A typical OCT camera used for scanning the OCT image is shown in figure 1.
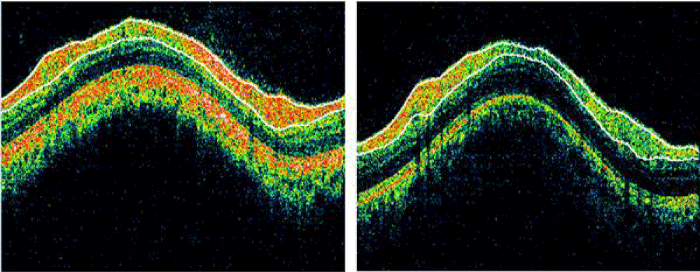
The OCT image of normal (left) and AD (right) taken by the OCT camera is shown in figure 2.
Figure 1: OCT Camera (Source: SGMC & RF).
Figure 2: OCT Image of normal (left) and AD (right) (Source: SGMC & RF).
Biomedical engineering technology
In the above section, OCT imaging system can be used for the diagnosis of AD. The examination of OCT images will open a wide area in the field of AD prognosis. If these images can be analysed easily, the diagnosis of the disease will be easier. For this purpose, the use of biomedical technology is very useful for the automatic analysis of OCT images. Therefore, soft computing techniques like neural networks, fuzzy logic, support vector machines and wavelet networks can be very useful for diagnosing the disease [11].
There are different neuropathology tests and techniques for diagnosing AD. Some of the most advanced tests include functional magnetic resonance imaging, positron emission tomography and single photon emission computed tomography techniques. These techniques are very expensive, and a common man cannot afford the expense. In this scenario, OCT imaging technique is very useful for diagnosing the disease, as it very reliable and economically good. With the help of OCT imaging and soft computing techniques, it may be possible to early diagnosis of the disease in a convenient way.
The recently emerging evidence supports the fact that the retina of the eye may provide an opening in the screening, early diagnosis, or monitoring of the treatment of AD. Currently, a definite diagnosis of AD can only be confirmed post-mortem and a premortem diagnosis of AD. Hence a newly reliable and efficient method should be developed to diagnose the disease with the advanced Biomedical Engineering technology using the aid of the OCT imaging technique. The AD confirmation test can be made with minimum cost and time. The early identification of AD can be made with the above-mentioned method reliably and effectively. As we know that this disease is progressing worldwide with no suitable diagnosis, an effective approach towards this can be made with a point of view to diagnose AD with the minimum effort, cost, and time.
We would like to express our gratitude to Sree Gokulam Medical College and Research Foundation, Trivandrum, India for providing the necessary OCT images for the preparation of this paper. Also, we are thankful to the professors Dr. K Mahadevan, Ophthalmology department, and Dr. Manoj P, Neurology department for their support in this research work.
- Sandeep CS, Sukesh Kumar A. A Review on the Early Diagnosis of Alzheimer’s Disease (AD) through Different Tests, Techniques and Databases. AMSE J. 2015; 76: 1-22.
- Ohno-Matsu K. Parallel findings in age-related macular degeneration and Alzheimer’s disease. Prog Retin Eye Res. 2011; 30: 217–238. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21440663
- Mapstone M, Dickerson K, Duffy CJ. Distinct mechanisms of impairment in cognitive ageing and Alzheimer’s disease. Brain. 2008; 131: 1618-1629. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18385184
- Krasodomska K, Lubinski W, Potemkowski A, Honczarenko K, Pattern electroretinogram (PERG) and pattern visual evoked potential (PVEP) in the early stages of Alzheimer’s disease. Doc Ophthalmol. 2010; 121: 111-121. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20549299
- Guo L, Duggan J, Corderio MF. Alzheimer’s disease and retinal neurodegeneration. Curr Alzheimer Res. 2010; 7: 3-14. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20205667
- Mei M, Leat S. Suprathreshold contrast matching in maculopathy. Invest Ophthalmol Vis Sci. 2007; 48: 3419-3424. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17591917
- Kesler A, Vakhapova V, Korczyn AD, Naftalive E, Neudorfer M. Retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Clin Neurol Neurosurg. 2011; 113: 523-526. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21454010
- Oliveira LT, Louzada PR, Mello FG, Ferreira ST. Amyloid-b decreases nitric oxide production in cultured retinal neurons:a possible mechanism for synaptic dysfunction in Alzheimer’s disease? Neurochem Res. 2011; 36: 163-169. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20936504
- Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL. Retinal Abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2007; 48: 2285-2289. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17460292
- Sandeep CS, Sukesh Kumar A. The Early Diagnosis of Alzheimer’s Disease Using Advanced Biomedical Engineering Technology”, Neurological Disorders and Imaging Physics. 2: Application to Autism Spectrum Disorders and Alzheimer's, IOP Expanding Physics, 2019.
- Sandeep CS, Sukesh Kumar A, Mahadevan H, Manoj P. Analysis of Retinal OCT Images for the Early Diagnosis of Alzheimer's. Disease”, Springer-Advances in Intelligent Systems and Computing book series (AISC). 2018; 749: 509-520.