More Information
Submitted: September 12, 2022 | Approved: September 26, 2022 | Published: September 27, 2022
How to cite this article: Sherief ST, Taye K. Congenital alveolar rhabdomyosarcoma - case report. Int J Clin Exp Ophthalmol. 2022; 6: 035-037.
DOI: 10.29328/journal.ijceo.1001048
Copyright Licence: © 2022 Sherief ST, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Rhabdomyosarcoma; Orbit; Neonatal; Alveolar; Rare disease
Abbreviations: ARMS: Alveolar Rhabdomyosarcoma; CT: Computed Tomography; RMS: Rhabdomyosarcoma; VAC/VTC regimen: Vincristine, Sctinomycin D and Cyclophosphamide
Congenital alveolar rhabdomyosarcoma - case report
Sadik Taju Sherief* and Kalekirstos Taye
Department of Ophthalmology, School of Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
*Address for Correspondence: Sadik Taju Sherief, Department of Ophthalmology, School of Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia, Email: [email protected]; [email protected]
Rhabdomyosarcoma is the most common soft tissue sarcoma of childhood and is very rare in the neonatal period. At this age, the alveolar type is a remarkably uncommon variety.
We report a 56 days old female with alveolar RMS of the right eye noted since the age of 7 days with fast progression and unfavorable prognosis.
Congenital alveolar RMS is an important cause of neonatal onset rapidly progressive proptosis. Early onset, alveolar type, and late diagnosis were poor prognostic factor.
Rhabdomyosarcoma (RMS) is the most common childhood soft-tissue mesenchymal tumor [1]. It accounts for ~50% of all malignant soft tissue tumors in the pediatric population and 45% of these occur in the head and neck region [2]. Nearly 10% of childhood RMS cases arise in the orbit which is considered a favorable anatomic site since most of the patients present with localized disease [3,4]. The mean age of onset is 7–8 years old, it is very rare at birth [5]. RMS occurrence during the neonatal period is a poor prognostic sign. [6-9].
RMS has four main histopathologic subtypes: embryonal, pleomorphic, alveolar, and botryoid. [10]. The embryonal type (50% - 70% of orbital RMS) frequently seen cells are bipolar cells with tapered cytoplasmic processes. [11]. A pleomorphic subtype, which virtually exclusively affects adult patients, has the worst prognosis [12]. The 20% - 30% of orbital RMS that is of the alveolar type are characterized by poorly defined aggregates of malignant cells that are poorly differentiated [10]. The botryoid appears to be a papillary-configuring variation of the embryonal type [10].
Histologically, most orbital RMS in children are generally embryonal or alveolar [5].
Alveolar RMS that is present at birth is known as congenital alveolar RMS, which is uncommon and appears to be clinically distinct from alveolar RMS in older children [3].
Neonatal Alveolar RMS (ARMS) of the orbit is an extremely rare condition according to the current literature [10,14]. Hence, we hereby report neonatal ARMS of the orbit presented with massive proptosis.
A 56-day-old Ethiopian female infant was referred to our department due to proptosis of the right eye. Initially, a small painless swelling was noted at age of 7 days. After two weeks the mass started to increase in size associated with protrusion of the right eye. She was transferred to the Tikur Anbessa specialty hospital where she was admitted for the pain and growing mass. She was given an intravenous steroid with an impression of capillary hemangioma. As there was no response for treatment, she was referred to our department. She was the first child and there was no similar illness in the family.
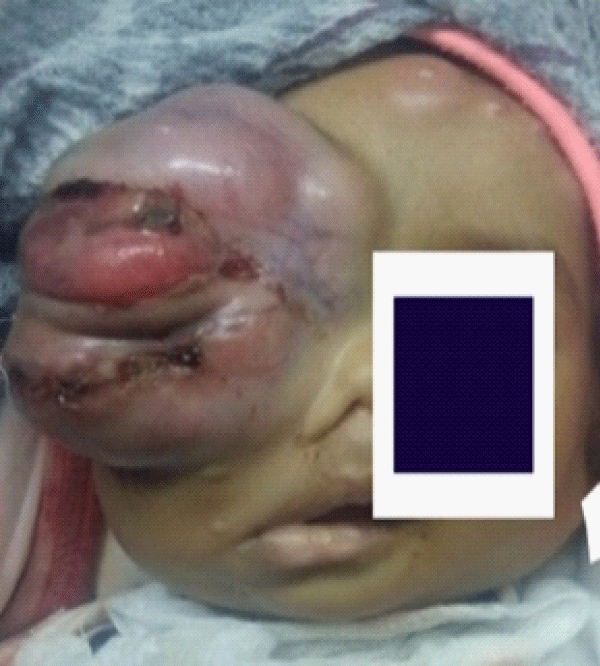
Upon examination in our department, we noted a tumor measuring 6 x 5 cm, tender, hyperemic and firm swelling hanging over the right side of the chec k was noted. The mass caused massive supero-temporal proptosis of the right eye (Figure 1). No nodules were seen. The other organ systems were normal in structure and function.
Figure 1: Clinical Photograph of the patient with proptosis and massive lesion in the right eyelid 5 days after the imitation of chemotherapy.
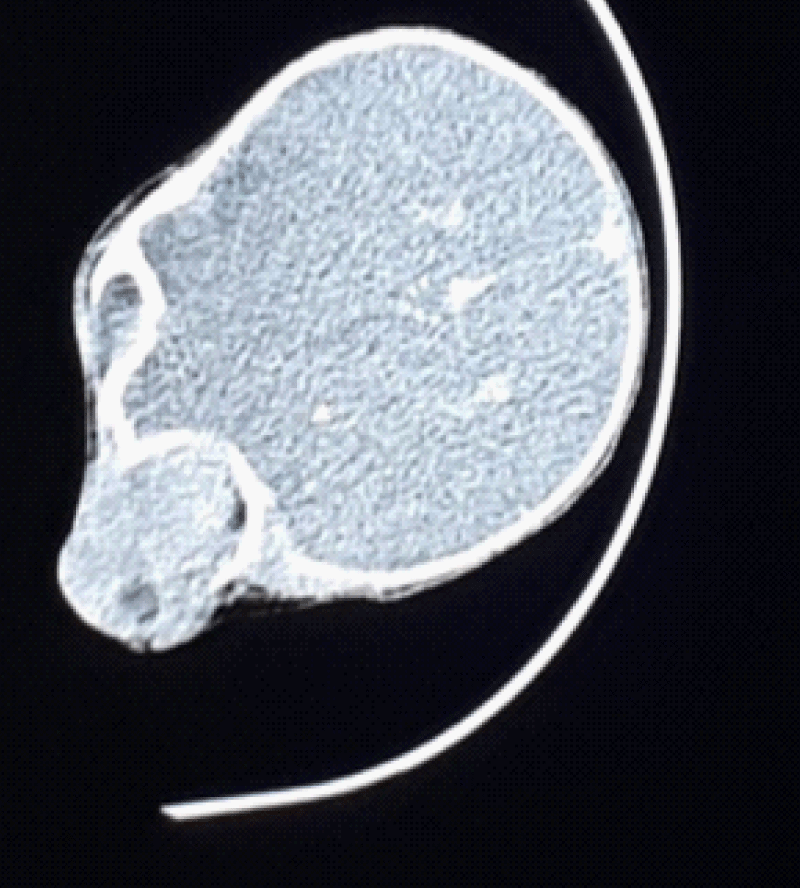
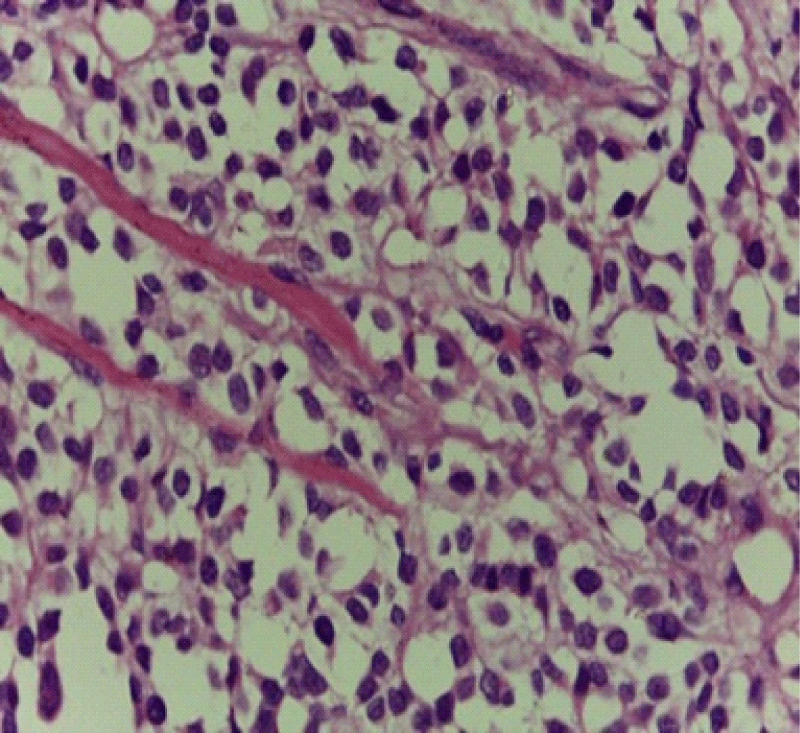
Systemic workup including complete blood count, urinalysis, serum electrolytes, creatinine level, liver function tests, abdominal ultrasound, and chest X-ray was unremarkable. Computed tomography (CT) scans showed a large irregular but well-circumscribed mass in the anterior and inferior of the right eyelid with retrobulbar extension (Figure 2), there was no lesion in the optic nerves and no metastasis suggestive of a malignant process. An incisional biopsy was taken directly overlying the tumor and a tissue sample was sent to Tikur Anbesa specialized hospital for histologic studies. The biopsy showed ill-defined aggregates of poorly differentiated malignant cells that are loosely arranged and separated into irregular ovoid spaces by thin fibrovascular septa (Figure 3). In this case, it was not able to perform an immunohistochemical examination. In addition to RMS, meningocele, orbital teratoma, hemangioma, and lymphangioma were considered differential diagnoses based on the patient’s history and clinical features at the time of presentation. The main difference from the rest of the differential diagnoses was the rapid progression of the swelling in this case.
Figure 2: Orbital CT Scan without contrast showed a large isodense mass filling the orbit and extending to the maxillary and ethmoidal sinus.
Figure 3: Aggregates poorly differentiated malignant cells that are loosely arranged and separated into irregular ovoid spaces by thin fibrovascular septa.
These findings confirmed that the diagnosis of the feature was consistent with alveolar rhabdomyosarcoma. Based on the Intergroup Rhabdomyosarcoma Study, this patient was classified as stage 3, group III, intermediate risk group, and accordingly, VAC/VTC regimen (vincristine, actinomycin D, and cyclophosphamide) was initiated, but died one week after initiation of the chemotherapy.
Neonatal significant proptosis is an extremely rare clinical condition. Meningocele, orbital teratoma, lymphangioma, hemangioma, and rhabdomyosarcoma are the differential diagnosis for neonatal proptosis [13]. As RMS is very rare neoplasia in neonates, accounting for only 2.24% of all RMS, there is limited literature available to review [14].
Usually, male predominance is observed in children with RMS. But our case, similar to other reported neonatal RMS cases, was female [9-12].
Occasionally RMS is associated with Li-Fraumeni syndrome, Noonan syndrome, neurofibromatosis type 1, hereditary retinoblastoma, Costello syndrome, and Beckwith-Wiedemann syndrome [15].
Embryonal RMS is the commonest histologic type and is usually seen in neonates and older children. [8,10,12]. In contrast to our case, presented at neonatal age with ocular involvement, ARMS is most commonly seen in older children and young adults involving the deep tissues of extremities [16]. The Head and neck, retroperitoneum, genitourinary tract, and extremities are the commonest sites of involvement of RMS [17]. The orbit is the commonest site of involvement for RMS within the head and neck [10,12]. Proptosis was the most frequent initial clinical sign of orbital RMS and two-thirds of orbital rhabdomyosarcoma have a tumor located in the superonasal part of the orbit [4,17]. Similarly, our patient was presented with proptosis but involving the inferior part of the orbit.
The diagnosis of RMS was made on the basis of biopsy and histopathological examination along with the CT Scan. Our patient had a tumor with a diameter greater than 5 mm, and no regional lymph node or distant metastasis was noted. Thus it was classified as T1NOMO. Regarding the Intergroup Rhabdomyosarcoma Study group, she had a localized gross tumor remaining after biopsy. Hence, the disease was classified as group III [18].
Current management of RMS includes surgery, irradiation, and chemotherapy depending on the stage [10-12]. In our case, the patient died while receiving chemotherapy, after incisional surgery. Similar to another report, in our case, we learned that the clinical management of RMS in patients under one year of age is a challenge [22].
Age under 12 months, delay in diagnosis, size of the tumor (> 5 cm), alveolar or undifferentiated histologic types nonorbital location, lymph node involvement, and presence of metastasis at presentation are poor prognostic factors of RMS due to the high risk of metastasis and poor response to various types of treatment modalities [19].
The alveolar morphology, neonatal age at presentation, tumor size > 5 cm, and delay in the presentation were the poor prognostic factors noted in our case.
Hence, Orbital RMS should be considered in a patient with rapidly progressive unilateral proptosis. And this unusual presentation of orbital rhabdomyosarcoma in a neonate prompted us to share our clinical experience.
Declaration
Ethical consideration: Ethical clearance was obtained from the Institute Ethics Committee Review of the Department of Ophthalmology of Addis Ababa University to publish the case report and written consent was obtained from the parents to use his clinical data and pictures for the report. The parents understand that their name and initials will not be published and due efforts will be made to conceal their identity in the pictures.
- Wexler LH, Helman LJ. Rhabdomyosarcoma and the undifferentiated sarcomas. In: Pizzo PA, Poplack DG, eds. Principles and Practice of Pediatric Oncology, 3rd ed. Philadelphia: Lippincott- Raven Publishers; 1997:799-829.
- Wurm J, Constantinidis J, Grabenbauer GG, Iro H. Rhabdomyosarcomas of the nose and paranasal sinuses: treatment results in 15 cases. Otolaryngol Head Neck Surg. 2005 Jul;133(1):42-50. doi: 10.1016/j.otohns.2005.03.023. PMID: 16025051.
- Owosho AA B Ch D, Huang SC Md, Chen S Mbbs, Kashikar S Dds, Estilo CL Dmd, Wolden SL Md, Wexler LH Md, Huryn JM Dds, Antonescu CR Md. A clinicopathologic study of head and neck rhabdomyosarcomas showing FOXO1 fusion-positive alveolar and MYOD1-mutant sclerosing are associated with unfavorable outcome. Oral Oncol. 2016 Oct;61:89-97. doi: 10.1016/j.oraloncology.2016.08.017. Epub 2016 Sep 6. PMID: 27688110; PMCID: PMC5097864.
- Jurdy L, Merks JH, Pieters BR, Mourits MP, Kloos RJ, Strackee SD, Saeed P. Orbital rhabdomyosarcomas: A review. Saudi J Ophthalmol. 2013 Jul;27(3):167-75. doi: 10.1016/j.sjopt.2013.06.004. PMID: 24227982; PMCID: PMC3770217.
- Huh W, Mahajan A: Ophthalmic oncology. In: Esmaeli B (ed): Ophthalmic Oncology. Boston, Springer, 2011,pp61–67.
- Ragab AH, Heyn R, Tefft M, Hays DN, Newton WA Jr, Beltangady M. Infants younger than 1 year of age with rhabdomyosarcoma. Cancer. 1986 Dec 15;58(12):2606-10. doi: 10.1002/1097-0142(19861215)58:12<2606::aid-cncr2820581209>3.0.co;2-t. PMID: 3779610.
- Salloum E, Flamant F, Rey A, Caillaud JM, Friedman S, Valteau D, Lemerle J. Rhabdomyosarcoma in infants under one year of age: experience of the Institut Gustave-Roussy. Med Pediatr Oncol. 1989;17(5):424-8. doi: 10.1002/mpo.2950170513. PMID: 2796858.
- Lobe TE, Wiener ES, Hays DM, Lawrence WH, Andrassy RJ, Johnston J, Wharam M, Webber B, Ragab A. Neonatal rhabdomyosarcoma: the IRS experience. J Pediatr Surg. 1994 Aug;29(8):1167-70. doi: 10.1016/0022-3468(94)90302-6. PMID: 7965528.
- Dillon PW, Whalen TV, Azizkhan RG, Haase GM, Coran AG, King DR, Smith M. Neonatal soft tissue sarcomas: the influence of pathology on treatment and survival. Children's Cancer Group Surgical Committee. J Pediatr Surg. 1995 Jul;30(7):1038-41. doi: 10.1016/0022-3468(95)90337-2. PMID: 7472928.
- Shields JA, Shields CL. Rhabdomyosarcoma: review for the ophthalmologist. Surv Ophthalmol. 2003 Jan-Feb;48(1):39-57. doi: 10.1016/s0039-6257(02)00415-0. PMID: 12559326.
- Terezakis SA, Wharam MD. Radiotherapy for rhabdomyosarcoma: indications and outcome. Clin Oncol (R Coll Radiol). 2013 Jan;25(1):27-35. doi: 10.1016/j.clon.2012.07.009. Epub 2012 Sep 16. PMID: 22990007.
- Sultan I, Qaddoumi I, Yaser S, Rodriguez-Galindo C, Ferrari A. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol. 2009 Jul 10;27(20):3391-7. doi: 10.1200/JCO.2008.19.7483. Epub 2009 Apr 27. PMID: 19398574.
- Singh AD, Traboulsi EI, Reid J, Patno D, Chapa J, Rodriguez R, Iben S, Schoenfield L. Orbital cyst: prenatal diagnosis. Ophthalmology. 2009 Oct;116(10):2042-42.e2. doi: 10.1016/j.ophtha.2009.06.032. PMID: 19800532.
- Russo I, Di Paolo V, Gurnari C, Mastronuzzi A, Del Bufalo F, Di Paolo PL, Di Giannatale A, Boldrini R, Milano GM. Congenital Rhabdomyosarcoma: a different clinical presentation in two cases. BMC Pediatr. 2018 May 15;18(1):166. doi: 10.1186/s12887-018-1128-5. PMID: 29764408; PMCID: PMC5953406.
- Godambe SV, Rawal J. Blueberry muffin rash as a presentation of alveolar cell rhabdomyosarcoma in a neonate. Acta Paediatr. 2000 Jan;89(1):115-7. doi: 10.1080/080352500750029185. PMID: 10677070.
- Sultan I, Qaddoumi I, Yaser S, Rodriguez-Galindo C, Ferrari A. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol. 2009 Jul 10;27(20):3391-7. doi: 10.1200/JCO.2008.19.7483. Epub 2009 Apr 27. PMID: 19398574.
- Dur˜aes G V, Jham B C, ,Mesquita A T M, et al. Oral embryonal rhabdomyosarcoma in a child: a case report with immunohistochemical analysis, Oral Oncology Extra. 2006;42 (3): 105–108.
- Pizzo PA, Poplack D, Rhabdomyosarcoma G. Principles and practices of pediatrics oncology. seventh ed. 2016. p798–822.
- Morgan E, Baum E, Breslow N, Takashima J, D'Angio G. Chemotherapy-related toxicity in infants treated according to the Second National Wilms' Tumor Study. J Clin Oncol. 1988 Jan;6(1):51-5. doi: 10.1200/JCO.1988.6.1.51. PMID: 2826715.