More Information
Submitted: 17 July 2019 | Approved: 09 August 2019 | Published: 12 August 2019
How to cite this article: de Sousa BA, Teixeira A, Salaroli C, Souza N, Gomes L. Wound architectural analysis of 1.8mm microincision cataract surgery using spectral domain OCT. Int J Clin Exp Ophthalmol. 2019; 3: 008-012.
DOI: 10.29328/journal.ijceo.1001020
Copyright Licence: © 2019 de Sousa BA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Cataract; Phacoemulsification; Spectral domain OCT; Self-sealing clear corneal incision; Microincision cataract system
Wound architectural analysis of 1.8mm microincision cataract surgery using spectral domain OCT
Benedito Antônio de Sousa1, Anderson Teixeira1-3*, Camila Salaroli2, Nonato Souza2 and Lucy Gomes4
1Department of Ophthalmology, Catholic University of Brasília, Brasília, Brazil
2Clinica de Olhos Teixeira Pinto, Brasília, DF, Brazil
3Department of Ophthalmology, Federal University of São Paulo, São Paulo, Brazil
4Gerontology Pos-Graduate Department, Catholic University of Brasília, Brasília, Brazil
*Address for Correspondence: Anderson Teixeira, SDS Bloco D no 27 sala 306, Brasília-DF-Brazil, ZIP# 70392-901, Brazil, Tel: +55 11 3825 7754; Email: [email protected]
Purpose: Analyze Microincision Cataract surgery wound using Fourier-Domain optical coherence tomography.
Setting: Medical School of Medicine, Catholic University of Brasilia, Brasília, Brazil.
Design: Prospective comparative observational study
Methods: Forty eyes were included in this prospective study divided in two groups: with contact lens (CL) and without contact lens (WCL). A line scan pattern of the corneal incisions were acquired using a Spectral domain OCT system immediately after the surgery, and at postoperative days 1, 7 and 30. Incisions were analyzed regarding length, location, angle, architecture, and anatomic imperfections.
Results: All incisions were located temporal or nasal superiorly. The average wound length was 1.28 + 0.18mm and the mean incision angle was 49 + 9 degrees. The average wound length of the WCL group mean was 1.24 + 0.17 mm and the mean incision angle was 51 + 8 degrees. Comparing groups for the length and the angle, the incisions measurements were not statistically significant. Anatomic imperfections were observed at the first day postoperative in 12 eyes for CL group and in 13 eyes for the WCL group. No patient presented endophthalmitis during the follow-up.
Conclusion: Epithelial imperfection was observed in two patients in the WCL group with spontaneous resolution. The CL group had the highest length and lowest angle of corneal incision. Using contact lens to prevent wound construction imperfection appears not to be a good option. Further studies using a greater number of patients with an architectural analysis of clear corneal incisions are needed to confirm these preliminary results.
Micro-incision cataract surgery (MICS) was developed in the last decades to increase the results in phacoemulsification surgery. It was derived from the traditional phacoemulsification cataract surgery incision called Clear Corneal Incision (CCI). CCI is the global choice technique for cataract surgery procedure, and during years it becomes a safe and effective [1]. The 1.8mm MICS may have some advantage comparing to traditional CCI wound, the advantages includes: reduce the risk for intraoperative anterior chamber instability, less incision bleeding during the surgery, higher structural stability of the anterior chamber, the magnitude of surgically induced astigmatism (SIA), easy in construction and les incidence of postoperative endophthalmitis [2]. Combined with the development of cold phaco technique and intraocular lens for ultra-small incision, greater achievements have been made to improve the cataract surgery quality. However, smaller incisions can produce complications if the incisions are too tight and result in excessive mechanical or thermal corneal trauma [3].
All surgical techniques and phacoemulsification tips cause some amount of wound trauma [4,5]. Clinical implications include difficult in wound sealing, increased use of sutures, wound edema, SIA, and prolonged healing [3,6].
The good quality resolution and faster acquisition of the images using anterior segment optical coherence tomography (AS-OCT) with the technology of spectral domain (SD-OCT) allows adequate screening for postoperative follow-up of the wounds. This exam analyzes the corneal incision with a non-contact method and was used to examine the architecture features of CCI in vivo after cataract surgery using CCI technique [7-13].
The objective of this study is to analyze MICS wound using Spectral-Domain optical coherence tomography after phacoemulsification surgery and to correlate them to surgical outcomes.
Randomly selected eyes were directed to cataract surgery having the MICS CCI performed. Patients participants were divided in two groups: 20 eyes were performed MICS CCI and after the surgery a contact lens (CL) with -0.50 diopters (1-Day Acuvue Trueye, Johnson and Johnson Vision Inc, Ireland, UK) was used for 24 hours as a tamponade band aid and 20 eyes were performed MICS CCI without contact lens (WCL). No stromal edema was performed after the surgery in any patient.
All surgeries were performed by one surgeon (AT) under topical anesthesia and CCI was performed superior temporally in the right eye and superior-nasally in the left eye. The incisions were made with a 1.8mm sapphire blade (Aurora Surgical Inc, St. Petersburg, FL, USA), Bausch and Lomb Stellaris PC System (Bausch and Lomb Surgical Inc., St Louis, MO) was the elect phacoemulsification using Burst mode ultrasound parameter with less than 0.5 minutes per surgery. Lens cortex was removed by automated irrigation and aspiration using standard setup. Foldable acrylic intraocular lens (Akreos MI60 AO, Bausch and Lomb Inc, St Louis, MO, USA) was injected into the capsular bag using a 1.8mm disposable implantation system; no incision tunnel enlargement was performed in any patient. The eyes were filled with BSS to normal pressure on palpation without leaking. Phacoemulsification and side-port incisions were not sealed with stromal hydration.
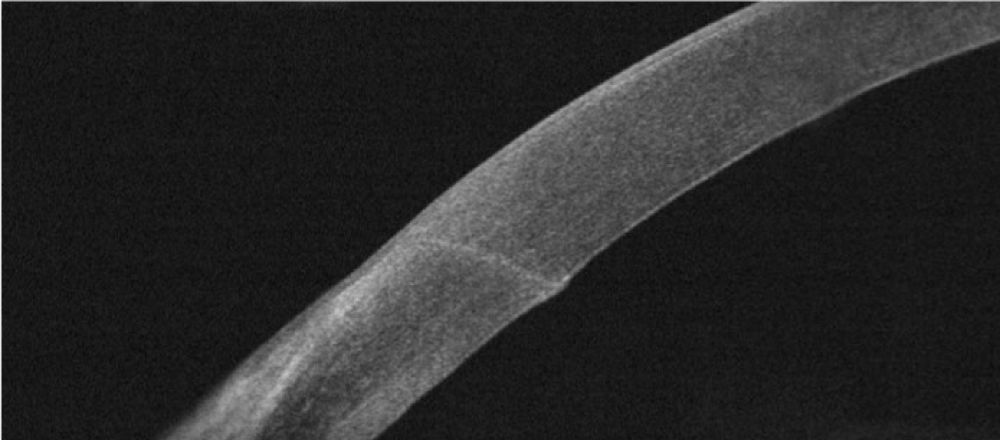
To study the incision morphology during the postoperative days (Immediately after the surgery and 1, 7 and 30 days) a corneal adaptor module (CAM) of SD-OCT system (RTVue, Optovue Inc., Fremont, CA, USA) was used. To measure the CCI a single line scan was positioned perpendicular to the limbus in all samples. Wound length (Figure 1), architectural deformations and angle between the corneal surface tangents were the following parameters studied using radial scans of the SD-OCT.
Figure 1: SD-OCT after cataract extraction showing the 1.8mm corneal incision.
To analyze statistically all saples a SAS V9.1 programming language (SAS Institute Inc., Cary, NC) was used. The data were analyzed by linear length, looking for shallow groove, incision angle, epithelial and endothelial gaping. Paired t-test was used to compare the different incision techniques and analysis of variance tested across days. Accepted level of significance for all tests was a p value of less than 0.05.
Forty eyes of 32 participants were enrolled and completed the study (10 males). Mean age was 65 + 7 years (range: 60 to 82 years). During the study no incision leakage or intraoperative complications were seen in any of the subjects. Hypotony, endophthalmitis or shallow chamber were no reported during the period of the study. Three patients did not complete the follow up and were excluded from to the study.
The average wound length of the CL group was 1.28 ± 0.18mm (range: 0.80mm to 1.70mm) and in WCL group was 1.24 ± 0.17mm (range: 0.83mm to 1.59mm). This was not significantly different (p = 0.17). The average angle of the incision relative to the tangent plane to the corneal surface was 49 ± 9 degrees (range: 26 to 70 degrees) for CL group and 51 ± 8 degrees (range: 31 to 73 degrees) for WCL group. There were no significant differences between groups (p = 0.16). Tables 1 and 2 show all post-operative average measurements for both groups with the analysis of variance between days.
| Table 1: Mean and standard deviation of FD-OCT measurements performed in the without contact lens group after cataract extraction (n=19 eyes). | ||||
| Length(mm) | Angle(degrees) | Epithelial gaping(mm) | Endothelial gaping(mm) | |
| Postoperative Period | ||||
| Day 0 | 1.31 ± 0.18 | 56 ± 8 | 0.01 ± 0.1 | 0.23 ± 0.12 |
| 1 day | 1.26 ± 0.14 | 54 ± 7 | 0.0 | 0.18 ± 0.13 |
| 7 days | 1.2 ± 0.12 | 52 ± 6 | 0.0 | 0.64 ± 0.09 |
| 30 days | 1.11 ± 0.16 | 41 ± 6 | 0.0 | 0.27 ± 0.08 |
| ANOVA | 0.89 | 0.43 | 0.71 | |
| Table 2. Mean and standard deviation of FD-OCT measurements performed in the with contact lens group after cataract extraction (n=18 eyes). | ||||
| Length(mm) | Angle(degrees) | Epithelial gaping(mm) | Endothelial gaping(mm) | |
| Postoperative Period | ||||
| Day 0 | 1.40 ± 0.16 | 51 ± 9 | 0.0 | 0.28 ± 0.14 |
| 1 day | 1.34 ± 0.15 | 53 ± 11 | 0.0 | 0.15 ± 0.14 |
| 7 days | 1.25 ± 0.17 | 51 ± 6 | 0.0 | 0.50 ± 0.10 |
| 30 days | 1.15 ± 0.17 | 41 ± 6 | 0.0 | 0.15 ± 0.06 |
| ANOVA | 0.89 | 0.24 | 0.54 | |
In vivo architecture features of MICS and CCI after cataract surgery
Epithelial gaping was observed only in 15% of the participants for the WCL group at the same day of surgery. No epithelial changes were observed after the first day postoperatively in both groups.
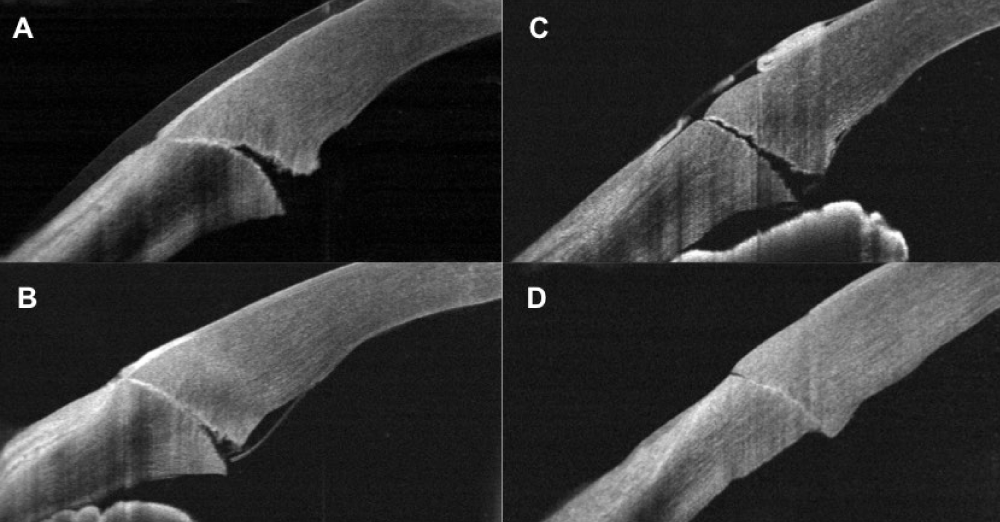
Endothelium gaping was observed in both groups. For the CL Group was observed in 25% of the participants at the same day of surgery, in 20% one day after the surgery and 10% seven day after the surgery. For the WCL group was observed in 45% at the same day of surgery, 65% at the first and seven day postoperatively. Two patients presented endothelial gaping at 30 days postoperatively in the WLC group. The average of endothelial gaping for CL group was 0.11+0.13mm (range: 0.0mm to 0.46mm) and for WCL group was 0.12+0.15mm (range: 0.0 mm to 0.64 mm), with no significant differences between groups (p=0.70). Descemet’s detachment and endothelial misalignment were observed in both groups (Table 3 and Figure 2) with a complete resolution at 30 days postoperatively only in the CL group.
| Table 3: Number of eyes with architectural changes observed using FD-OCT. | ||
| Contact lens group | Without contact lens group | |
| Descemet’s detachment | 5 | 9 |
| Endothelial misalignment | 0 | 1 |
| Loss of coaptation | 0 | 0 |
| Endothelium gaping | 4 | 9 |
| Epithelium gaping | 0 | 2 |
Figure 2: Complications of 1.8mm corneal incision after cataract surgery observed with SD-OCT imaging. A) Day one postoperatively, contact lens group clear corneal incision: endothelium gaping. B) One day postoperatively, without contact lens group clear corneal incision: endothelium gaping. C) Zero day postoperatively without contact lens group clear corneal incision: loss of coaptation. D) Seven day postoperatively without contact lens group clear corneal incision: endothelium misalignment.
The study used AS-OCT to show the applicability and usefulness of this method to analyze the advantage of 1.8mm MICS incision technique. As a non-invasive exam and combined with easy analysis tools, the OCT became an excellent method to quantitatively analyze structural changes in corneal wound architecture after phacoemulsification in patients who submitted a cataract surgery. The analysis technique was simple to perform, multiple key parameters measured (incision angle, incision length, wound changes) and marking the area for analytical calculation was reproducible. Furthermore, spectral domain AS-OCT has enabled clinicians to obtain images with higher resolution and higher scan rate, allowing more accurate measurements [14-18].
Alterations of the internal surface of the cornea wound was described before [6,14]. Whikert et al., in the study, using electronic microscopy, showed no remarkable cell loss or descemet membrane rearing when the incision was made without out manipulation, in cases involving phaco tip, I/A hadpicies and IOL injector manipulations conspicuous areas of endothelial cell loss surrounding each incision were present. However, a qualitative finding reported in our sample appears to align that the wound construction defect are related to the instruments manipulation and/or IOL injection. To maintain the integrity and health of the eye the surgeons need to considerer the best wound construction.
Endothelial cell damage after MICS and standard-incision cataract surgery has been evaluated, and incision size was not found to be a direct factor to influence endothelial cell loss [18]. In contrast, Mahdy, et al. and Park, et al. also founded statistically significant endothelial cell loss using MICS technique, especially with cases of nuclear hardness cataract. With increasing cataract density, there was a need for more manipulation to remove the lens and can be a factor to change the architecture of the wound [18-20].
Sapphire blades for incisions are better in terms of sharpness, quickness, and ease of use. They allow the surgeon to create a more reproducible and consistent corneal incision tunnel [21]. Because sapphire blades require minimal force, reproducible incisions can be constructed easily with little distortion of corneal tissue. Initiating incorrectly the plane incision (e.g. too long tunnel) can create a movement limitation in the anterior chamber (AC) and excessive manipulation can increase the structural damage of the wound. In our series, the mean lengths were 1.28mm and 1.24mm for WCL and CL, respectively. We believed tunnels between 1.0mm to 1.5mm are save enough to maintain the mobility of the instruments in the AC without structural wound damage.
No stromal hydration was performed in this study; we believe MICS are too small for hydration and swelling caused by the incision. In our concept, the angle of the incision shown to affect the self-sealing properties of the wound and small angles are more effective in providing a self-sealing condition [7,14,22,23]. In this study, the angle appeared to be shallow enough to create sealing in both groups (average angle 49 degrees for WCL group and 51 degrees for CL group). We found no significant difference between groups at the end of this study.
The use of contact lens appears not be useful to avoid structural changes in our sample. We found a high percentage of endothelial gaps or Descemet membrane detachments in the WCL group compared to CL group (p = 0.70). The literature shoes the incidence of endothelial wound gaping varies between 25% to 70%, depending on the postoperative day [9,12,24,25]. There are studies describing some possible explanations to endothelial wound gaping such as phacoemulsification instruments sizing or excessive ultrasound power, corneal stromal edema or endothelial damage, [12,13,26] and some authors added IOP fluctuation as a factor [22,24]. No epithelial defect was observed in the CL group, as far as we know, external gaps can be found in CCI with a fast resolution [22,24-26] and can be associated to intraocular pressure fluctuation and stromal hydration [9,13,24].
This study provided accurate measurement of the MICS wound in vivo using SD-OCT high-resolution. All images were clearly visible after the phacoemulsification procedure without significant motion error analyzing.
In conclusion, MICS corneal incisions in both groups imaged with SD-OCT presented internal gaps in the immediate postoperative period with a spontaneous resolution and the use of contact lens do not prevent any internal gap, even our results were no statist significant or the sample were too small. The technology of SD-OCT allowed immediate postoperative non-contact evaluation of wound incision, and provided high-resolution measurements for evaluating the incision’s morphology. This information can be used to perform corneal incision techniques in order to diminish the risk of gaping, which can be related to increase risk of postoperative complications and visual rehabilitation.
- Lee KM, Kwon HG, Joo CK. Microcoaxial cataract surgery outcomes: comparison of 1.8 mm system and 2.2 mm system. J Cataract Refract Surg. 2009; 35: 874-880. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19393887
- Long DA, Monica ML. A prospective evaluation of corneal curvature changes with 3.0- to 3.5-mm corneal tunnel phacoemulsification. Ophthalmology. 1996; 103: 226-232. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8594506
- Weikert MP. Update on bimanual microincisional cataract surgery. Curr Opin Ophthalmol. 2006; 17: 62-67. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16436926
- Wilczynski M, Drobniewski I, Synder A, Omulecki W. Evaluation of early corneal endothelial cell loss in bimanual microincision cataract surgery (MICS) in comparison with standard phacoemulsification. Eur J Ophthalmol. 2006; 16: 798-803. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17191184
- Bourne RRA, Minassian DC, Dart JKG, Rosen P, Kaushal S, et al. Effect of cataract surgery on the corneal endothelium; modern phacoemulsification compared with extracapsular cataract surgery. Ophthalmology. 2004; 111: 679-685. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15051198
- Weikert MP, Wang L, Barrish J, Dimalanta R, Koch DD. Quantitative measurement of wound architecture in microincision cataract surgery. J Cataract Refract Surg. 2012; 38: 1460-1466. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22814053
- Schallhorn JM, Tang M, Li Y, Song JC, Huang D. Optical coherence tomography of clear corneal incisions for cataract surgery. J Cataract Refract Surg. 2008; 34: 1561-1565. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18721720
- Torres LF, Saez-Espinola F, Colina JM, Retchkiman M, Patel MR, et al. In vivo architectural analysis of 3.2 mm clear corneal incisions for phacoemulsification using optical coherence tomography. J Cataract Refract Surg. 2006; 32: 1820-1826. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17081864
- Calladine D, Packard R. Clear corneal incision architecture in the immediate postoperative period evaluated using optical coherence tomography. J Cataract Refract Surg. 2007; 33: 1429-1435. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17662437
- Can I, Bayhan HA, Celik H, Bostanci CB. Anterior segment optical coherence tomography evaluation and comparison of main clear corneal incisions in microcoaxial and biaxial cataract surgery. J Cataract Refract Surg. 2011; 37: 490-500. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21333873
- Lyles GW, Cohen KL, Lam D. OCT-documented incision features and natural history of clear corneal incisions used for bimanual microincision cataract surgery. Cornea. 2011; 30: 681-686. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21242779
- Fukuda S, Kawana K, Yasuno Y, Oshika T. Wound architecture of clear corneal incision with or without stromal hydration observed with 3-dimensional optical coherence tomography. Am J Ophthalmol. 2011; 151: 413-419e1. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21236408
- Xia Y, Liu X, Luo L, Zeng Y, Cai X, et al. Early changes in clear cornea incision after phacoemulsification: an anterior segment optical coherence tomography study. Acta Ophthalmol. 2009; 87: 764-768. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19548882
- Teixeira A, Salaroli C, Filho FR, Pinto FT, Souza N, et al. Architectural analysis of clear corneal incision techniques in cataract surgery using Fourier-domain OCT. Ophthalmic Surg Lasers Imaging. 2012; 43(Suppl): S103-108. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23357317
- Lee H, Kim EK, Kim HS, Kim TI. Fourier-domain optical coherence tomography evaluation of clear corneal incision structure according to blade material. J Cataract Refract Surg. 2014; 40: 1615-1624.
- Wylegala E, Teper S, Nowinska AK, Milka M, Dobrowolski DE. Anterior segment imaging: Fourier-domain optical coherence tomography versus time-domain optical coherence tomography. J Cataract Refract Surg. 2009; 35: 1410-1414. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19631129
- Can I, Bayhan HA, Celik H, Bostancı CB. Anterior segment optical coherence tomography evaluation and comparison of main clear corneal incisions in microcoaxial and biaxial cataract surgery. J Cataract Refract Surg. 2011; 37: 4 90-500. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21333873
- Mastropasqua L, Toto L, Vecchiarino L, Di Nicola M, Mastropasqua R. Microcoaxial torsional cataract surgery 1.8 mm versus 2.2 mm: functional and morphological assessment. Ophthalmic Surg Lasers Imaging. 2011; 42:114-124. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21323269
- Mahdy MA, Eid MZ, Mohammed MA, Hafez A, Bhatia J. Relationship between endothelial cell loss and microcoaxial phacoemulsification parameters in noncomplicated cataract surgery. Clin Ophthalmol. 2012; 6: 503-510. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22536044
- Park JH, Lee SM, Kwon J-W, Kim MK, Hyon JY, et al. Ultrasound energy in phacoemulsification: a comparative analysis of phaco-chop and stop-and-chop techniques according to the degree of nuclear density. Ophthalmic Surg Lasers Imaging. 2010; 41: 236-241. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20307043
- Marshall J, Trokel S, Rothery S, Krueger RR. A comparative study of corneal incisions induced by diamond and steel knives and two ultraviolet radiations from an excimer laser. Br J Ophthalmol. 1986; 70: 482–501. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3013283
- Taban M, Rao B, Reznik J, Zhang J, Chen Z, et al. Dynamic morphology of sutureless cataract wounds--effect of incision angle and location. Surv Ophthalmol. 2004; 49 (Suppl 2): S62-72. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15028481
- Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, et al. Optical coherence tomography. Science. 1991; 254: 1178-1181. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1957169
- McDonnell PJ, Taban M, Sarayba M, Rao B, Zhang J, et al. Dynamic morphology of clear corneal cataract incisions. Ophthalmology. 2003; 110: 2342-2348. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14644716
- Fine IH, Hoffman RS, Packer M. Profile of clear corneal cataract incisions demonstrated by ocular coherence tomography. J Cataract Refract Surg. 2007; 33: 94-97. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17189800
- Fukuda S, Kawana K, Yasuno Y, Oshika T. Repeatability and reproducibility of anterior chamber volume measurements using 3-dimensional corneal and anterior segment optical coherence tomography. J Cataract Refract Surg. 2011; 37: 461-468. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21333870